It has been decades since DCA, also known as dichloroacetate, was first used to treat infants with mitochondrial disorders. Cellular organelles known as mitochondria are essential for the generation of energy necessary for numerous cellular processes. DCA increases mitochondrial activity in infants born with faulty mitochondria and has a variety of consequences on cellular metabolism.
Because cancer cells often divide fast and exhibit aberrant cellular processes and energy needs, the effects of DCA cancer treatment on cellular processes and metabolism have generated expectations for a targeted treatment against cancer cells. It’s possible that DCA’s capacity to modify cellular fuel, such as sugar, may be used to starve and kill tumor cells. Cancer research centers throughout the globe are doing significant studies on the effects of DCA. The majority of this study’s funding comes from the public, and it’s still in its infancy. because no pharmaceutical firm has indicated an interest in conducting expensive research initiatives on a medicine that is easily accessible, affordable, and so unlikely to generate financial profit.
The drug administration
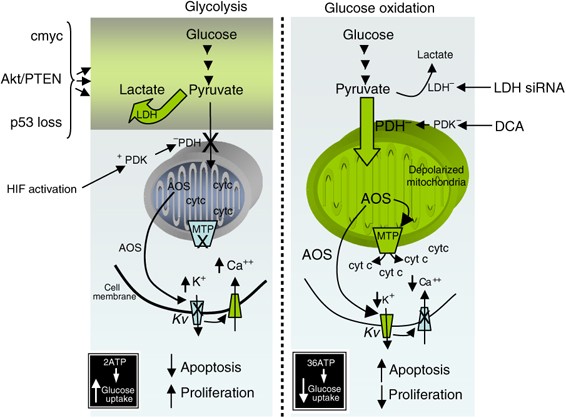
As long as the common distal routes remain necessary and particular to cancer cells, the issue of ‘proximal’ heterogeneity may be addressed by targeting additional ‘distal’ pathways that combine many proximal signals. To achieve a glycolytic phenotype and high apoptosis resistance, the unique metabolism of most solid tumors combines several nearby pathways and leads to mitochondrial remodeling (where energy production regulation and apoptosis convergence).
A rising body of data suggests that the mitochondria may be the main targets in cancer therapies, rather than just bystanders during cancer formation. Dichloroacetate (DCA), a mitochondria-targeting small chemical that permeates most tissues following oral treatment, may correct this cancer-specific metabolic reprogramming. Positron emission tomography (PET) imaging may also be used to monitor DCA’s molecular and direct metabolic response to tumor glucose absorption, non-invasively and in the future. An oncologist’s experimental therapies paradigm might be shifted by metabolic methods such as these.
Many different cancers might benefit from chloroacetate’s treatment. Since many signaling pathways and oncogenes are implicated in the development of a glycolytic phenotype and resistance to apoptosis, as well as the fact that most carcinomas have hyperpolarized or remodeled mitochondria, as well as the fact that the majority of solid tumors show increased glucose uptake on PET imaging, it appears that DCA cancer treatment may be effective against a wide range of tumor types and types in general.
Treatment effects
DCA’s anti-cancer effects have only been shown in preclinical studies in non-small cell lung cancer, glioblastoma, and malignant breast, endometrial, and prostate cancers. Some cancers, such as sarcoma, lymphoma, and oat cell lung carcinoma, do not have hyperpolarized mitochondria, suggesting that DCA may not be helpful in these circumstances. Cancers like recurrent glioblastoma and advanced lung cancer, which have few or no therapy alternatives, should be at the top of the priority list for research.
When using DCA in clinical studies, it is important to keep a close eye on neurotoxicity and define explicit dosing reduction procedures. For the study of cancer patients, the pharmacokinetics of the drug will need to be established. DCA monotherapy should be tested in clinical trials, either as a single agent or in direct contrast to other medicines, based on preclinical results.